How Precision Neuro Obtained a 510(k) for an Implant BCI
A rare Class II path for an invasive brain implant where most competitors still face the long, data‑heavy PMA route
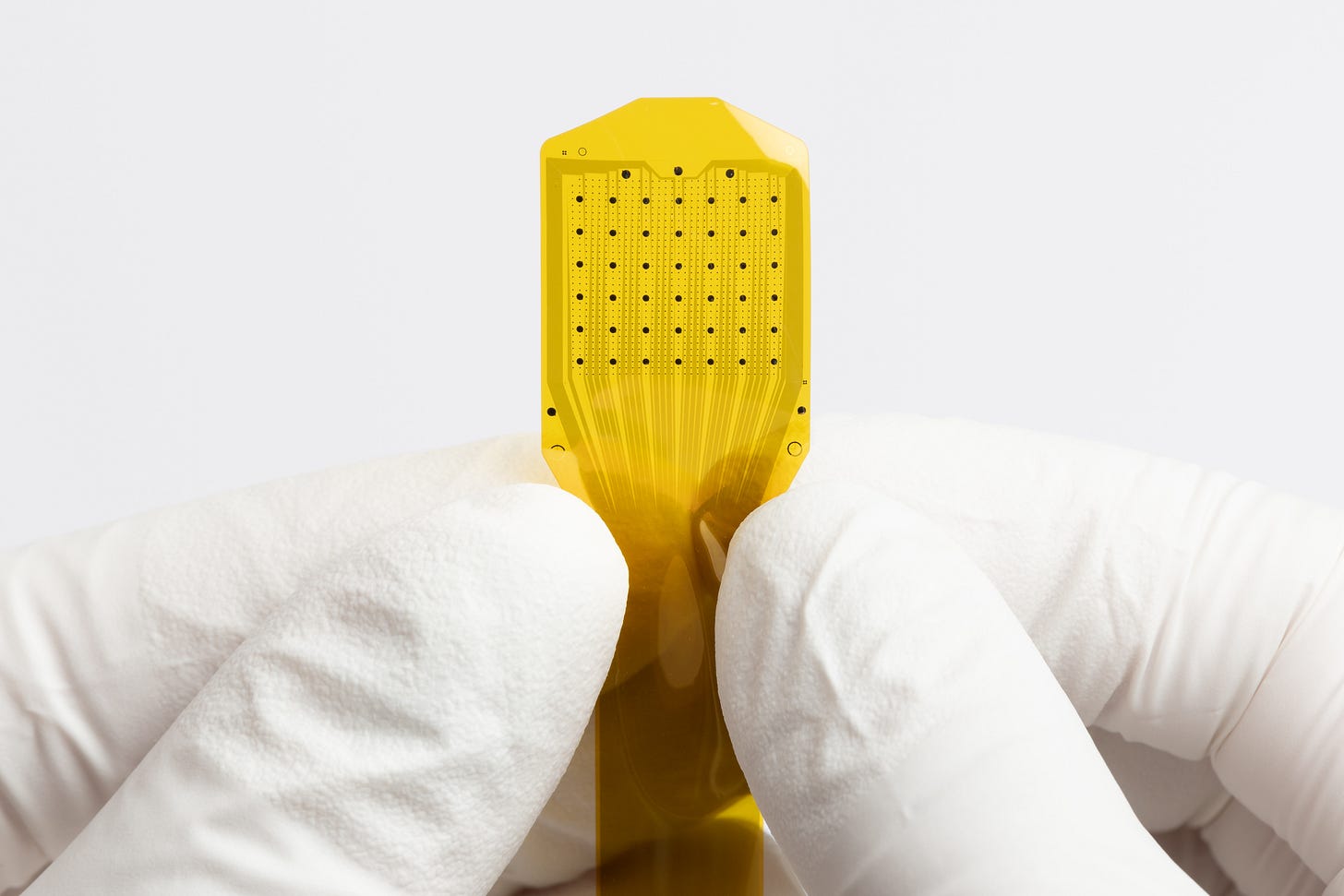
On 30 March 2025 the U.S. FDA cleared Precision Neuroscience’s Layer 7‑T Cortical Interface under 510(k) number K242618. The letter lists the device as a Class II cortical electrode (product code GYC) and confirms substantial equivalence to earlier technology, meaning Precision can commercialise without a Premarket Approval (PMA) or De Novo.
Read the full 510(k) submission and the FDA’s notes here:
Why a 510(k) is so surprising for an implant
Invasive neuro‑devices that penetrate or reside on the brain surface normally fall into Class III, which pushes them into the costly PMA regime (e.g., deep‑brain stimulators, fully implantable neuroprostheses). FDA’s own overview of neurological devices makes that hierarchy clear: Class II covers “moderate‑risk” tools like aneurysm clips, while Class III captures high‑risk implants. U.S. Food and Drug Administration FDA’s guidance on implanted BCIs echoes this, warning sponsors to be prepared for chronic‑toxicity, carcinogenicity and other long‑term data usually associated with PMAs.
What the clearance covers—and what it does not
Cleared hardware only (passive electrode & insertion tools).
Valid for ≤ 30 day implantation; permanent BCI indications (e.g., prosthetic control) will still require an IDE study and probably a PMA or De Novo for the full closed‑loop system.
Software decoding algorithms packaged outside the electrode itself would need their own cybersecurity and SaMD documentation in a future submission.
Precision’s classification pivot & a tight indication‑for‑use
The company avoided Class III by fitting Layer 7 neatly into an existing Class II regulation—21 CFR 882.1310, Cortical electrode. The rule covers electrodes “temporarily placed on the surface of the brain for stimulating or recording electrical activity,” and it carries special controls rather than a PMA burden.
Precision mirrored the language in 882.1310: “temporary (< 30 day) use… recording, monitoring and stimulation of electrical signals on the surface of the brain… open or burr‑hole procedures.” By avoiding claims of long‑term implantation or closed‑loop prosthetic control, the company kept the intended use within the historical scope of subdural ECoG grids, short‑term seizure‑mapping arrays and other already‑cleared devices.
Crafting the Predicate Argument
Primary predicate: Ad‑Tech Subdural Electrodes (K191186); Same regulation, identical intended use, conventional silicone‑platinum construction—provides the core legal bridge.
Reference predicate: NeuroOne Evo Cortical Electrode (K192764): Demonstrates FDA familiarity with polyimide thin‑film arrays, the main material difference between Layer 7 and the Ad‑Tech device.
Ad‑Tech Medical Instrument’s subdural strips and grids, cleared under 510(k) K191186 in January 2020, sit squarely in 21 CFR 882.1310 (cortical electrode, Class II, product‑code GYC). The family includes multiple geometries, strip, grid, dual‑sided inter‑hemispheric, multi‑strip and mapping grids, each built from a silicone elastomer substrate into which platinum/iridium contacts are embedded and routed through insulated lead‑wires to a connector. The devices are supplied sterile, single‑use and have no active electronics.
The FDA cleared Ad‑Tech electrodes for temporary implantation (< 30 days) on the cortical surface “with recording, monitoring and stimulation equipment for the recording, monitoring and stimulation of electrical signals on the surface of the brain.” Clinical use cases called‑out in the 510(k) summary include localisation of epileptogenic foci and functional brain mapping, exactly the diagnostic window neurosurgeons already recognize.
Those indications, route of administration (open craniotomy or burr‑hole placement) and passive electrical‑conductor technology match the regulatory language Precision adopted for Layer 7. In its own 510(k) summary, Precision explicitly states that “the proposed intended use for Layer 7‑T and the intended use for the Ad‑Tech Subdural Electrodes are identical.”
From a technological‑characteristics standpoint, Layer 7 diverges only in form factor (polyimide thin‑film array, smaller contact diameters, higher channel count) while relying on the same noble‑metal electrode chemistry and passive cabling concept. FDA accepted Precision’s bench, biocompatibility and GLP animal data as sufficient evidence that these deltas “do not raise new questions of safety or effectiveness.” In other words, Ad‑Tech’s well‑established silicone‑platinum platform provided the core legal bridge that let Precision argue substantial equivalence and avoid the heavier PMA pathway.
Read the full 510(k) submission and the FDA’s notes here:
Testing checklist that sealed equivalence
ISO 10993 biocompatibility for ≤ 30‑day implantables.
IEC 60601‑1 / ‑1‑2 electrical safety & EMC on the complete cabled system.
Flex‑fatigue & tensile tests (> 1 million cycles) on the thin‑film array.
Porcine 1‑ and 6‑week subdural studies confirming no extra inflammatory response versus predicate.
Sterilisation validation (EtO, ISO 11135) and packaging shelf‑life (ASTM D4169).
All of those data sets are referenced in the K242618 summary as evidence that the new materials and form‑factor do not raise new questions of safety or effectiveness.
Regulatory choreography: What Precision did right
Pre‑submission (Q‑Sub) negotiation: secured FDA agreement on classification, predicate choice and test matrix before spending on studies.
Single, well‑chosen primary predicate: no stretching of intended use; avoided “split‑predicate” pitfalls.
Clear, limited indications: no performance claims beyond the historical envelope of cortical grids.
Robust but proportionate test battery: matched to special‑control expectations rather than PMA depth.
Rapid interactive review: submitted on 27 Feb 2025, cleared in just over a month, reflecting a clean dossier.
Lessons for other BCI innovators
Scope first, science second: The regulatory fight can be won or lost at the indication‑for‑use line; design yours to fit an existing regulation if one exists.
Leverage the ecosystem: Decades of epilepsy‑mapping grids created a robust family of Class II predicates. Precision showed how to ride that precedent.
Document every delta: Higher channel counts or novel substrates are permissible within 510(k) so long as bench, biocomp and animal data close the risk gap.
Plan for the long game: The current clearance covers the passive electrode hardware only. Any future closed‑loop, at‑home neuroprosthetic claims will almost certainly require an IDE and a higher‑risk pathway.






Was looking for more details on this. Thank you for breaking it down!